Intravenous & Irrigation Solutions
BAXTER SOLUTIONS, ALWAYS AT HAND
Intravenous (IV) solutions for fluid therapy and drug reconstitution are the lifeline of a hospital, and clinicians depend on them to be of high quality and readily available at all times. By offering critical IV solutions in closed-system, flexible containers and a commitment to supply continuity, Baxter provides that assurance and frees clinicians to focus on caring for their patients. Providers can also rely on Baxter's irrigation solutions being at their disposal when needed.

RIGOROUS QUALITY ASSURANCE
Baxter's IV solutions are manufactured in controlled facilities and environments using processes and equipment that are licensed and in compliance with EU and UK Good Manufacturing Practice (GMP) requirements for sterile medicinal products. Prior to market release, samples from each batch are subjected to multiple chemical, microbiological, physical and other tests in accordance with the marketing authorization for this product. These controls and tests ensure that products are safe, effective and in compliance with the licensed product specifications.

CONTINUAL INVESTMENT IN MANUFACTURING AND INFRASTRUCTURE
Baxter is dedicated to ensuring a consistent and sustainable supply, without interruption, to hospitals and patients everywhere we serve. We have made significant investments in our manufacturing capacity and distribution networks and have implemented regional and global diversification strategies to create deliberate manufacturing redundancies to help mitigate potential localised solution supply disruptions.

PRIORITISING FLUID MANAGEMENT
Recognising the crucial role of fluid therapy in patient care, and the need to avoid complications by giving the right fluid at the right time based on an individual patient’s physiologic condition, we offer a full selection of large-volume parenteral solutions in Viaflo flexible containers and the Equilibria Fluid Optimisation Program to help clinicians use them to maximise clinical effectiveness. When delivery of these solutions is guided by the insights provided by our Starling Fluid Management Monitoring System, we enable clinicians to take an informed approach to fluid management.
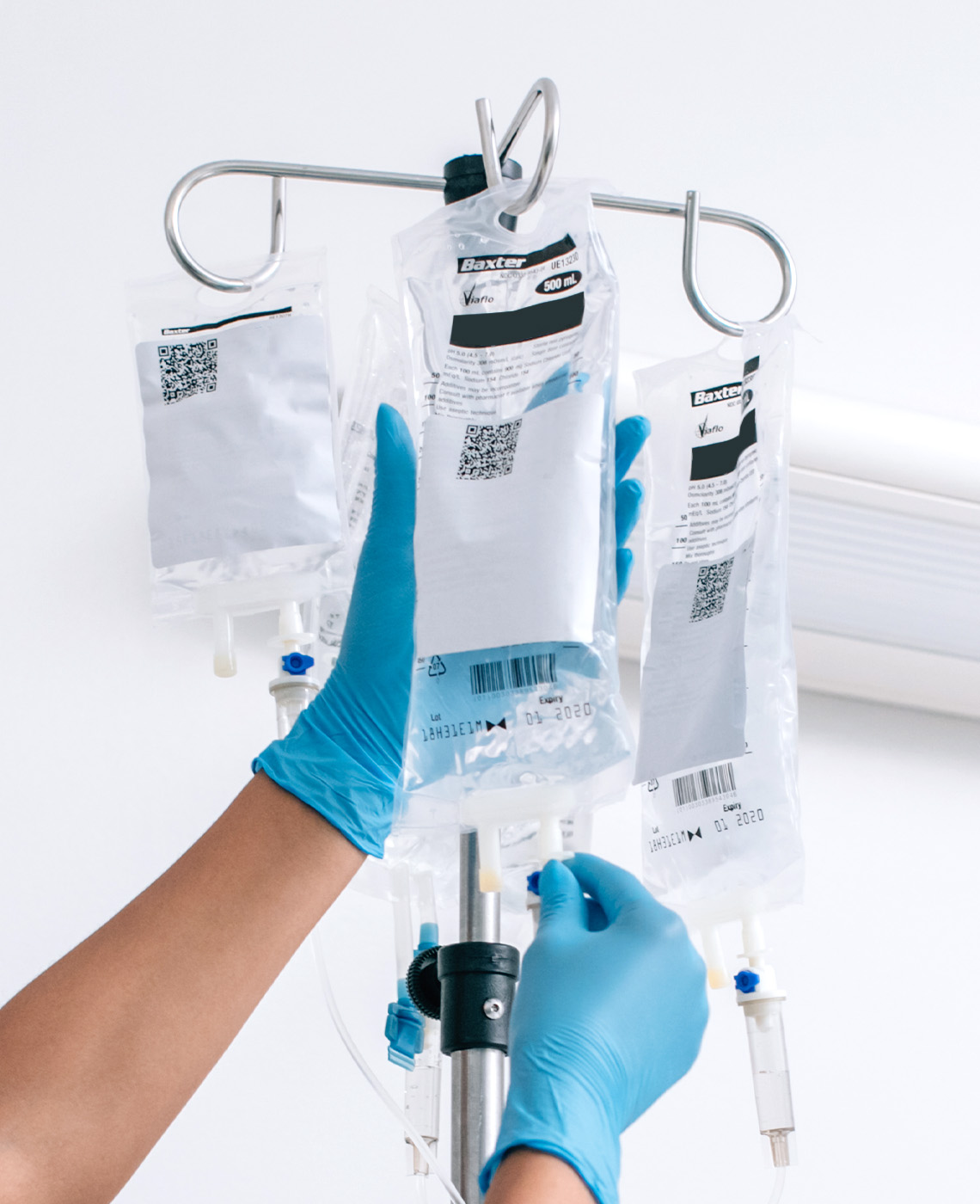
LARGE-VOLUME PARENTERAL SOLUTIONS TO MEET PATIENT NEEDS
When hospitalised patients cannot receive fluid orally or enterally, it must be delivered intravenously. Baxter provides large-volume parenteral solutions for resuscitation, replacement and maintenance scenarios.
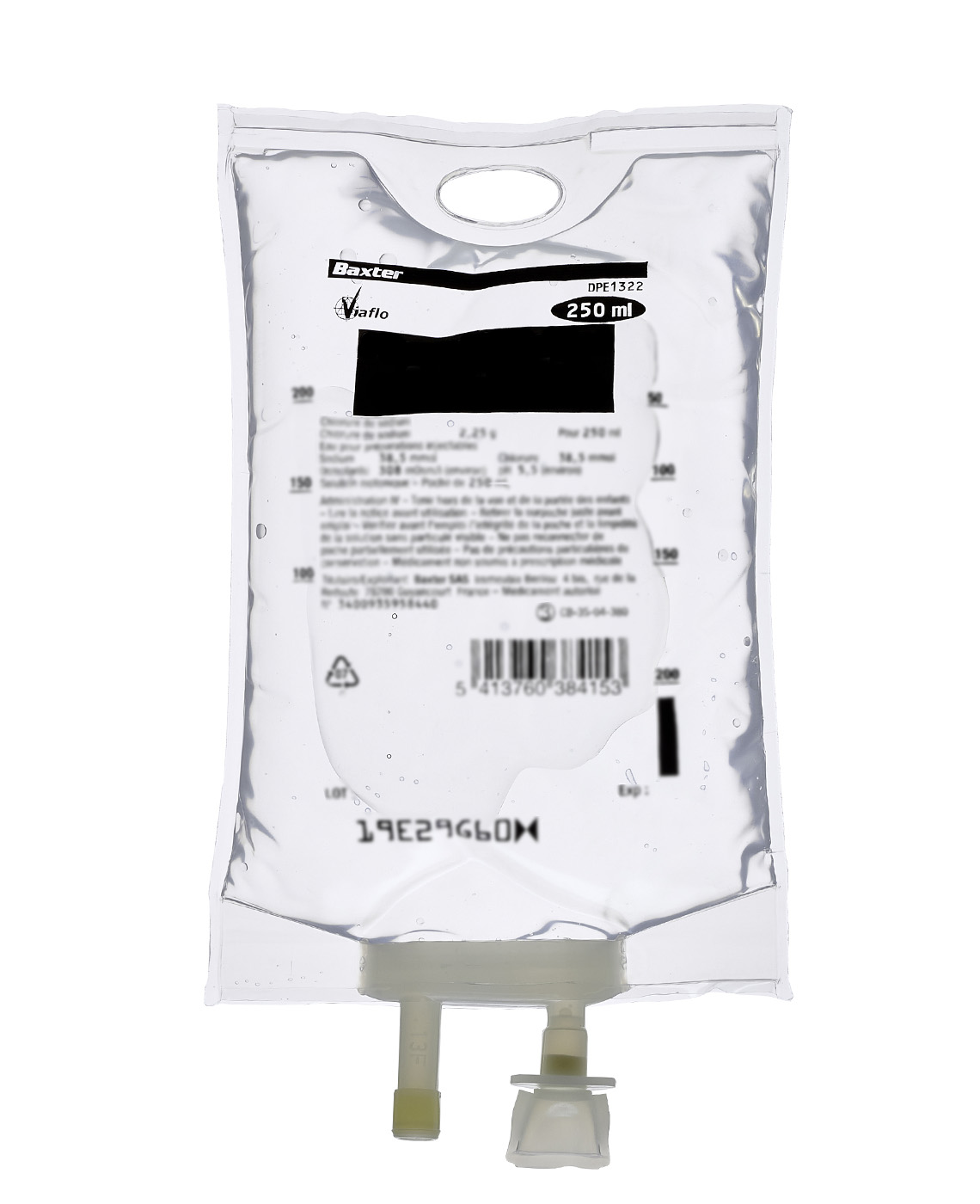
DRUG RECONSTITUTION
Baxter offers a range of diluents for drug reconstitution at the point of care or in the pharmacy. These solutions are provided in small volume parenteral Viaflo containers ranging in size from 50 ml to 250 ml. Closed-system Viaflo containers are free of latex, polyvinyl chloride (PVC) and Di(2-ethylhexyl) phthalate (DEHP).
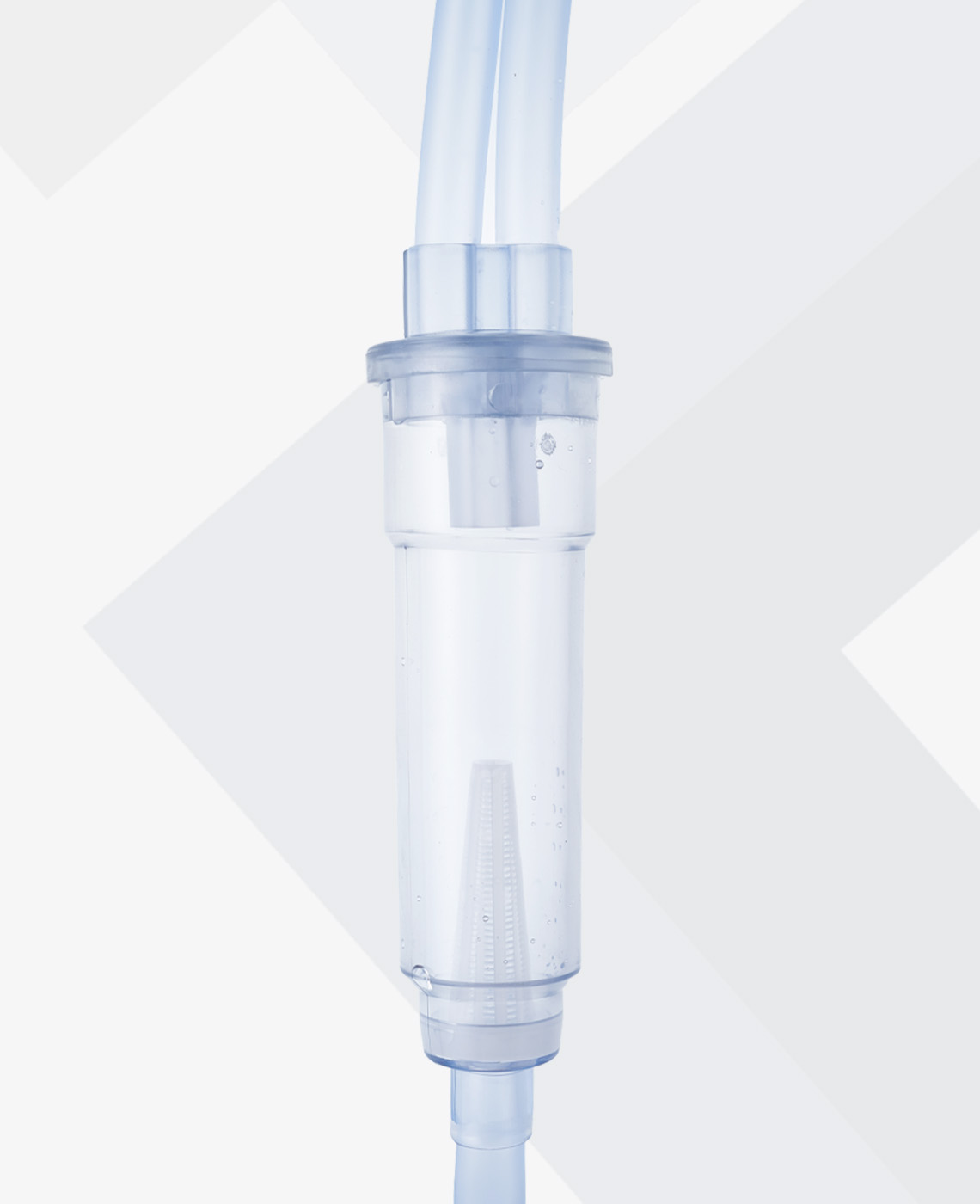
IRRIGATION SOLUTIONS AND SETS
Not intended for IV injection, Baxter’s sterile irrigation solutions may be used to wash or rinse body cavities, tissues or wounds, or medical devices and equipment. These solutions are housed in plastic pour bottles or in a range of flexible containers.
Baxter also provides selected irrigation sets to support a wide range of applications. These sets are designed to improve visibility and flow control, minimise touch contamination during spiking and enable appropriate flow rates. Certain sets incorporate a bubble trap in the drip chamber to avoid air bubbles in the scope view, providing clarity during surgery.
SV STARLING Monitor — Non-invasive haemodynamic monitoring system.
Intended purpose: The Starling SV system, with NIBP and SpO2 functionality, is intended for professional use in healthcare facilities. It is a portable non-invasive haemodynamic cardiac output monitor. It monitors and displays a patient's cardiac output (CO) in l/min with a non-invasive blood pressure (NIBP) function that non-invasively measures and displays blood pressure (diastolic, systolic and mean) and heart rate, and an SpO2 function that non-invasively measures and displays blood oxygen saturation (SpO2). The device displays the corresponding haemodynamic parameters based on measurements or measurement calculations already incorporated in the Starling SV.
Class IIa device. Notified body: MEDCERT - Germany (CE 0482).
Please refer to the instructions for use for information necessary for proper use.
Carefully read the instructions in the package insert.
Date of revision: 31 March 2020.
Baxter, Equilibria, Starling and Viaflo are trademarks of Baxter International Inc. or its subsidiaries.